Here are the descriptors, causes & possible fixes to the off-flavours from yesterday's meeting
Acetaldehyde (green apple)
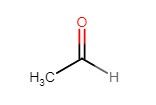
- green apple/dry cider
- acetaldehyde is a precursor to ethanol from glucose
- is found in every beer to some extent
- also caused by oxidation of ethanol
- lager yeasts can cause higher acetaldehyde
to avoid:
- sanitation
- use a healthy yeast pitch
- oxygenate wort (esp. at high gravity)
- Let the yeast clean up after themselves, avoid racking off yeast cake until fermentation is complete
- patience- wait until fermentation is complete after reaching terminal gravity
- avoid allowing O2 into beer after fermentation
Papery (trans 2-nonenal):
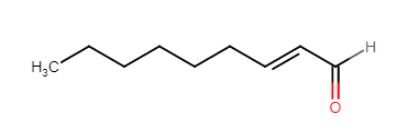
- the papery off-flavor occurs when beer is oxidized. It can also present itself as a cardboard flavor or even as a sweet stone/sherry flavour at lower concentrations.
- typically formed in packaging when stored at elevated temperatures (above 40°F) for any period of time due to the typically higher amount of dissolved oxygen present in beer after packaging.
- it’s very hard to remove all oxygen from beer – especially during packaging. Reduce oxygen inclusion at packaging
- the easiest and most important way to stave off oxidation is the proper storage of beer at cold temperatures.
- oxidation is a thermodynamically-driven process ∴ temperature plays a critical role in the shelf-life; keep beer cold and it’ll last longer
- a 2-4x decrease in freshness is seen for every 10°C/18°F increase in storage temperature.
to avoid:
- Purge bottles/kegs with CO2 before filling to remove air.
Solvent (ethyl acetate):
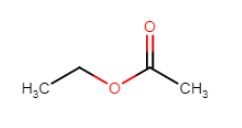
- Ethyl acetate is the most common acetate that is produced by brewing yeast, which has a flavor threshold of around 2PPM, and starts out with a fruity or pear-like flavor, but can end up tasting solvent-like >30 PPM. The style of beer being made defines the concentration and types of acetates that are desired in the resultant beer.
- Increasing the fermentation temperature will allow more fruity flavors to emerge from the beer, while decreasing the temperature will prevent these flavors, of which acetates are a factor.
- healthy yeast starter decreases the resultant acetates, while a weak starting yeast will produce more. This is why it is important that the timing of your yeast starter be good. If the yeast is not ready in time for pitching, and some other yeast gets colonised into the beer, it can have an additional acetate lacing effect.
- ethyl acetate can be produced in offensive levels by Brettanomyces when exposed to oxygen.
- ethyl acetate is a metabolic dead-end, meaning that there is not a known pathway for breaking it down; once it’s in your beer, it’s in your beer.
to avoid
- healthy yeast starter
- lower fermentation temperature helps reduce ester formation
Diacetyl (buttery)
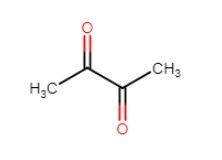
- buttery or butterscotch aroma
- flavour threshold between 10 & 40 ppm
- affects mouthfeel @high ppm slick/oily
- is more detectable towards end of glass due to high temperature as beer warms up
- is formed from two sources: during fermentation & infection
- diacetyl is a precursor to compounds (acetoin and butanediol) which are much less intense in taste and aroma ∴ almost imperceptible
- diacetyl occurs naturally during fermentation
- diacetyl rises to > 100 ppb in first few days of fermentation, drops 50% a day to low levels
- wort deficient in FAN can lead to elevated diacetyl
- can also be formed by spoilage (esp in presence of air)
how to eliminate?
- use high FAN malt
- healthy yeast pitch
- following end of airlock activity, allow a diacetyl rest @ ~70°F for 24-72 h
DMS (dimethyl sulphide)#
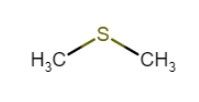
- flavour/aroma descriptor: a corn/creamed corn-like flavour/aroma
- threshold ~ 35 ppb
- typically higher in lagers than ales
- usually considered to be due to malts (like pilsner)
- however it originates in brew kettle from degradation of S-methyl methionine (amino acid)
to reduce
- evaporates out of wort- to reduce it forming simply leave the kettle lid off during boil
- rapid cool/whirlpool can also reduce: rapid cool means that no heat to form DMS & no boil to drive off the compound
Light struck
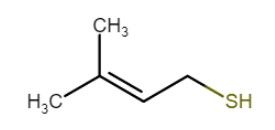
- the name is self-explanatory; it’s caused caused by light interacting with the beer
- Blue/UV (350-500 nm) light causes the reaction
- forms free radicals from iso-humulones (hop compounds) which interact with sulphur containing amino acids to form 3-Methyl-2-buten-1-thiol (3-MBT), a similar chemical found in skunk spray
to avoid
- avoid using green or clear glass to store beer (reduces blue/UV light)